Water Chemistry for Brewers: Creating Classic European Beer Styles

This is the second installment in a three-article series discussing water chemistry information and best practices for brewers. Read the first article, “Water Chemistry: What Every Commercial Brewer Needs to Know” here »
Want to brew classic European styles of beer? Here’s what you need to know about matching the water in the Old World capitals that invented them.
If you’re a brewer, you’ve almost certainly heard the term “Burtonizing.” But do you understand exactly why, when, and how to do it? According to Merriam-Webster, burtonizing refers to the process of hardening brewing water by “adding gypsum or certain salts especially for the purpose of approximating the flavor” of beers produced in the English town of Burton-on-Trent. Burton’s sought-after pale, hoppy beers earned it a prized reputation by the 12th century; by the 1800s, the original home of pale ale had fashioned itself into the epicenter of British brewing.
Just as pre-modern farmers and chefs – in the relative absence of imports or contemporary technology or technique – primarily relied on and perfected foods that grew naturally in the terroir around them, the pre-20th century brewers who created the classic styles of Europe maximized their efficiency and end results by tailoring their recipes around how the acidity of their grains would interact with the alkalinity and mineral composition of their local water sources.
Writes Ian Honsey in The Oxford Companion to Beer, “The suitability of Burton water for brewing lies in its extreme hardness, particularly in terms of calcium and magnesium sulfates. These so-called gypseous waters promote protein coagulation during boiling, allow high hop-rate usage, and promote yeast growth; the result being the clear, sparkling ale for which Burton became famous.”
Though their water sources vary considerably, the basic story reads similarly in Pilsen, Dublin, and other continental cities strongly associated with a certain style of brew. Today, brewers making them elsewhere often try to replicate these original water profiles to produce beers to style that become indistinguishable to those from their point of origin.
Here are some key things to know before you attempt to brew a classic pale ale, pils or dry Irish stout.
Wort pH and Water Alkalinity
“The primary interest to brewing is the pH of the wort during mashing. Factors such as water alkalinity and mash grist composition have greater effect on mashing pH than the starting pH of the raw water,” says brewing water engineer Martin Brungard. In other words, the pH of the mash is incredibly important, and it’s therefore important to correctly manage the raw water to match your grain bill when working to reach your mash pH target. In preparing your water, although the pH in your liquor tank might not matter much, the mineral content (or hardness) of that water does, as does your overall grain bill, because the two will combine to create the right environment for all the grain-based enzymes in the mash to do the work in creating the desired flavor profile.
Brungard explains, “During mashing, calcium and magnesium in the brewing water react with phosphatic compounds (phytins) in the malt to produce acids that neutralize the brewing water’s alkalinity.” This reaction between the minerals in the water and the phytins in the malt effectively acidifies the mash while also reducing the hardness of the water, giving it less buffering capacity. The balance (positive or negative) of the water alkalinity here is known in the brewing world as Residual Alkalinity (RA) and is a key factor in maintaining the proper pH of the mash.
Brungard says brewers should aim for a mash pH between 5.2 and 5.8, so as not to hinder its enzymatic processes. As a rule of thumb, pale beers favor low RA water because the mash pH finds a comfortable spot in that range. The opposite can be said for dark beers, whose acidic grains can unfavorably lower the wort pH and reduce activity of the mash enzymes, possibly resulting in a beer that’s inappropriately acidic, tart or sharp.
“As the acid content of the mash’s grain bill increases, the mashing water RA must also rise proportionally to maintain the mash pH,” writes Brungard.
To adjust the residual alkalinity of your brewing water, there are a few options. In lowering the RA, heating or boiling the water can greatly soften its hardness and decrease its alkalinity. The rise in temperature causes the pH to rise and the bicarbonate in the water to precipitate as calcium carbonate.
When raising the RA, John Palmer and Colin Kaminsky, the authors of Water, A Comprehensive Guide for Brewers, caution, “It is difficult to add more alkalinity to a soft water without adding significant hardness or sodium as well. It is often a case of two steps forward, one step back.” This is especially notable with sodium, as it is flavor positive and can noticeably impact the sensory profile of your finished product.
What does all of this mean? In practical terms, it’s highly important to consider the flavor contributions of the minerals in your water, and what impact additions or removals of those minerals will have on your finished beer.
Flavor Ions
Luckily, the water profiles of historic brewing cities and regions can give us a roadmap to mastering the proper mix of minerals and ions in your brewing water. Once you have the desired style of beer outlined and the grain bill calculated, it’s time to consider whether any flavor ions – notably calcium, magnesium, bicarbonate (alkalinity), sodium, chloride and sulfate – need to be added to approximate the water profile you seek.
From the Bru’n Water website:
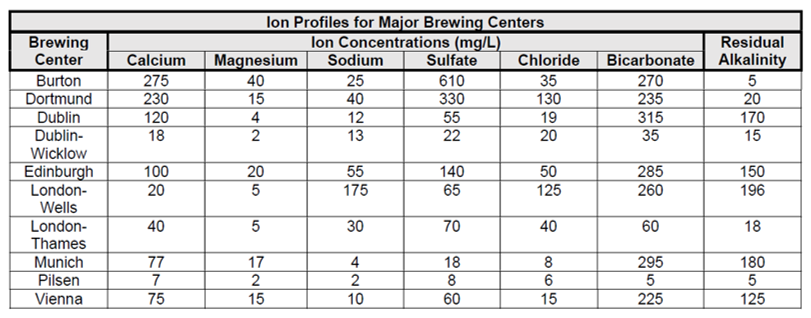
Comparing these profiles to water composition reports from your brewery’s water source will give you an idea of what and how much you might need to add or reduce in matching these profiles. While doing so, it’s important to know that the mineral makeup of a water source can change over time. Further, given that brewers have been modifying water for hundreds of years, brewers in those historic locations may have treated their own supply. Therefore, the provided parameters don’t paint the entire picture but, rather, sketch out a close approximation. Looking at a few key places on this list can give insight to the impact and importance the particular brewing water has for the styles that originated there.
Burton Pale Ales
Palmer writes in his book How to Brew that compared to London, Burton’s calcium and sulfate levels are “remarkably high, but the hardness and alkalinity are balanced to nearly the degree of Pilsen.”
He says high sulfates and low sodium content produce “an assertive, clean hop bitterness” that when compared with London, are “paler, but much more bitter.”
That said, Brungard warns that the Burton water profile in the above chart was estimated at a specific, highly mineralized aquifer whose relative concentrations of ions fluctuate throughout the year. Brewing at those concentrations may be extreme and produce notes of sulfur.
As if to illustrate the variability of Burton’s brewing water over time and space, Mitch Steele writes in IPA: Brewing Techniques, Recipes and the Evolution of India Pale Ale that its earliest breweries drew water from shallow (approx. 30 ft) wells close to the river Trent; As the river grew polluted, brewers increasingly dug deeper and farther away where the water was found to have three times more gypsum and half the calcium carbonate as the original wells.
Pilsen Pilsners
According to Palmer and Kaminsky’s co-authored text, “Recreating a pilsner with vastly different water is one of the greatest challenges a brewer can undertake.”
That’s because every ingredient and decision that goes into this pale Czech style rests on the soft surface and groundwater of Pilzen, which has low minerality and low alkalinity. Using only base malts to reach proper mash pH, Palmer likens pilsner to the “soft rich flavor of fresh bread” derived from an absence of sulfate that “provides for a mellow hop bitterness that does not overpower the soft maltiness.”
Dublin Stouts
“Famous for its stout,” begins Palmer in How to Brew, “Dublin has the highest bicarbonate concentration of the cities of the British Isles, and Ireland embraces it with the darkest, maltiest beer in the world. The low levels of sodium, chloride and sulfate create an unobtrusive hop bitterness to properly balance all of the malt.”
As opposed to breweries in the rest of Dublin, which draw from the city’s typically hard, alkaline water, Guinness’ St James Gate brewing water historically flows from the neighboring Wicklow Mountains.
“Dry Irish stout is quite a different stout than other stouts and porters,” Brungard says. “Guinness has sort of an acidic bite because they brew with what amounts to rainwater. For most porters and stouts you want that alkalinity because all those dark grains are very acidic. With Guinness, those roasty malts become richer and fuller so the chocolate and coffee notes come through.”
Free eBook: Leveraging Data-Driven Fermentation Performance Management
 Can fermentation management be improved, as a process? This eBook explores, in detail, how fermentation performance data analysis helps elevate product and business outcomes in a modern brewery, whether brewpub, microbrewery or regional craft brewer.
Can fermentation management be improved, as a process? This eBook explores, in detail, how fermentation performance data analysis helps elevate product and business outcomes in a modern brewery, whether brewpub, microbrewery or regional craft brewer.
You will learn:
- Day-by-day performance considerations – learned through the extensive examination of real-time fermentation tank data.
- Key recommendations from the Precision Fermentation science team at each major step of fermentation – “Day zero” (i.e. before you pitch your yeast), the first 24 hours, and day two through the end of fermentation.
- Best practices – Activity to watch out for, broken down by each key measurement – Dissolved oxygen, gravity, pH, pressure, internal/external temperature, and conductivity.
- Key findings that can help you solve problems and improve your results.

